XeF6 + 2H2O —-> XeO2F2 + 4HF Hello friends! We are here with another interesting compound. In this article, we will discuss various properties of XeO2F2. So, Keep reading…
Valence Electrons
The electrons in an atom revolve around its center, also known as the nucleus. Each electron carries a negative charge and has specific energy associated with it. The amount of energy of the electron increases as the electron moves away from the nucleus. Therefore, in an atom, the electrons housed farthest from the nucleus have the highest energy, and are known as the valence electrons. The valence shell i.e. the outermost shell houses the valence electrons that also participate in chemical bonding.
Octet Rule
As stated earlier, the atoms form chemical bonds using their valence electrons. However, the number and type of bond formed by an atom also depend upon these electrons present in the outermost shell. Usually, every atom tries to obtain the electronic configuration of its nearest noble gas to become stable. As all the noble gases, except helium, have 8 electrons in the outermost shell, the atoms of other elements also try to obtain eight electrons in their valence shell. This is known as the octet rule. This concept was put forth by Walther Kossel and Gilbert N. Lewis and lays the foundation of all the other concepts related to an atom viz. hybridization, molecular geometry, etc.
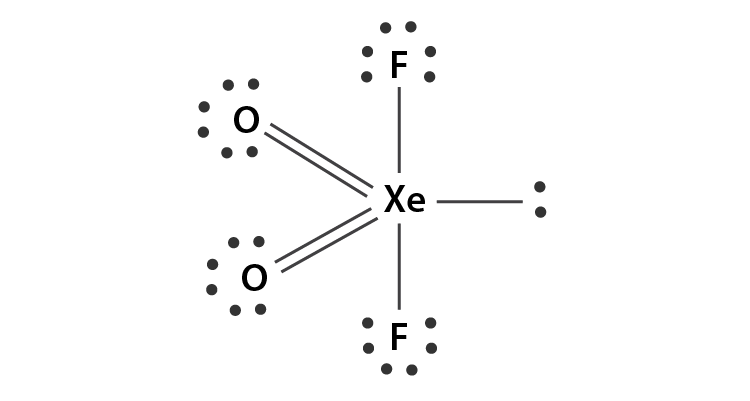
XeO2F2 Lewis Structure
The Lewis structure of an atom is a simplified representation of its atomic structure with its nucleus and valence electrons. It illustrates the arrangement of electrons in an atom. Here, electrons are represented with dots and a nucleus with the atomic symbol of that atom. The bond between two atoms is represented through a line. The Lewis structure of XeO2F2 is:
Looking at the Lewis structure of XeO2F2, it can be deciphered that all the atoms have attained their octet. Xenon, being a noble gas, already had eight valence electrons. Also, both fluorine and oxygen atoms that were lacking one and two electrons, respectively, have become stable by attaining octet. However, you may ask here that if Xenon already had eight electrons then why did it still form bonds with other atoms? You are right, this is impossible for most atoms. However, Xenon and other noble gases are an exception as they have vacant d-orbitals to accommodate the extra electrons. Xenon can expand its octet and accommodate more than eight electrons in its valence shell owing to the presence of vacant 5d orbitals.
Drawing Lewis Structure of XeO2F2
We will now draw the Lewis structure of XeO2F2 step by step: • First of all, we will calculate the number of valence electrons for each of the individual atoms present in one molecule of XeO2F2. For Xenon, a group 18 element, Number of valence electrons = 8 For Oxygen, group 16 element, Number of valence electrons = 6 Hence, for 2 oxygen atoms, the Total number of valence e– = 12 Similarly, for Fluorine, A group 17 atom, Number of valence electron = 7 Hence, for 2 fluorine atoms, Total number of valence e– = 14 Therefore, for XeO2F2 molecule, Total number of valence electrons = 34 • Now, we will choose a central atom for this molecule. Usually, the least electronegative and most stable atom is chosen for this purpose. In the present case, Xenon being the most stable atom is chosen as the central atom. • Next, we will link all the participating atoms to the central atom with a single bond. This is to evaluate if more electrons are required by any of the participating atoms. If this is so then further arrangements are made to complete its octet.
• From the above figure, it is clear that the octet for Xenon and fluorine atom is complete. However, each of the oxygen atoms still requires one more electron that can be provided by allowing the formation of a double bond between Xenon and oxygen atoms. • After this step, the octet for all the participating atoms is complete and the central atom is left with 4 bond pairs and one lone pair. • Hence, the final lewis structure of XeO2F2 is drawn as follows:
Formal Charge
The formal charge for a molecule is calculated to evaluate the stability of its Lewis structure. Although, it is a hypothetical concept but helps us determine the correctness of our derived structure. It is given by the formula: Formal Charge (FC) = Number of valence e– in an atom – Number of nonbonding e–– 1/2 (Number of bonding e–) The zero value of formal charge for a molecule indicates stability. We will now calculate the formal charge for the XeO2F2 molecule by calculating the formal charge for each of its atoms. For Xenon atom Number of valence electrons = 8 Number of non-bonding electrons = 2 Number of bonding electrons = 12 Therefore, Formal charge = 8 – 2 – ½(12) = 0 For Fluorine atom Number of valence electrons = 7 Number of non-bonding electrons = 6 Number of bonding electrons = 2 Therefore, Formal charge = 7 – 6 – ½(2) = 0 For Oxygen atom, Number of valence electrons = 6 Number of non-bonding electrons = 4 Number of bonding electrons = 4 Therefore, Formal charge = 8 – 4 – ½(4) = 0 As the formal charge on each of the individual atoms is zero. Therefore, the total formal charge on the XeO2F2 molecule also becomes zero. Hence, the Lewis structure that is drawn above for the XeO2F2 molecule is correct.
Molecular Geometry of XeO2F2
The molecular geometry of a compound is predicted using the postulates of Valence Shell Electron Pair (VSEPR) Theory. This theory states that the geometry of a molecule depends upon the number of bond pairs and lone pairs of electrons present on the central atom of that molecule. Basically, the idea is that all the electrons are negatively charged and as like charges repel each other these electrons also repel each other. This extent of repulsion is traced by the VSEPR theory to determine the shape of a molecule. The VSEPR theory further states that the extent of repulsion is different between bonding and non-bonding electron pairs. As the nonbonding electrons are free to move, the repulsion force between these electrons is maximum. Further, as the bonding electrons are already bound to two atoms, the freedom of movement is restricted due to which the force of repulsion between these electrons is minimized. Hence, there are two types of geometry for any molecule. One is the electron geometry that is predicted based on the bonding atoms and the other is the molecular geometry that also considers the role of lone pair of electrons in determining the geometry of a molecule. Based on the VSEPR theory we can determine both the electron geometry as well as the molecular geometry of a molecule by calculating the number of bond pairs and lone pairs of electrons present on the central atom of that molecule. In the case of XeO2F2, we have already calculated that the central atom i.e. Xenon has 4 bond pairs of electrons and one lone pair of electrons. Now, we can determine the geometry of XeO2F2, through the chart based on the postulates of the VSEPR theory as given below.
Therefore, the electron geometry of the XeO2F2 molecule is trigonal bipyramidal while the molecular geometry of this molecule is a see-saw. Also, the bond angles present between different atoms are 91o, 105o, and 174o, respectively.
XeO2F2 Hybridization
When a molecule is formed the orbital of similar energies also combine to form an entirely different orbital. This process is known as hybridization and was first put forth by Linus Pauling in 1931. The VSEPR theory states that the hybridization of a molecule can be calculated through the steric number which is given by the following formula: Steric No. = Number of sigma (σ) bond on central atom + lone pair on the central atom Therefore, for the XeO2F2 molecule, the steric number can be calculated using the above formula, as follows: Steric number for the XeO2F2 molecule = 4 + 1 = 5 We can now determine the hybridization state for the XeO2F2 molecule by using the following table: Therefore, the hybridization state for the XeO2F2 molecule is sp3d or dsp3. Also, we can determine the hybridization state by looking at the accommodation of the bonding electrons in the orbital of the central atom. In the case of the XeO2F2 molecule, we know that the hybridization state of the Xenon atom is sp3. However, being a noble gas, it still has a vacant d-orbital at its disposal. Therefore, in-ground state the hybridization of Xenon atom is written as:
In the excited state, the p electrons jump into the d sub-shell and the hybridization is written as:
The four excited electrons bond with two oxygen and two fluorine atoms and the remaining two electrons are left as lone pair. Therefore, the hybridization state of the XeO2F2 molecule is sp3d.
Polarity of XeO2F2
In the case of the XeO2F2 molecule, polarity exists owing to the electronegativity difference between the participating atoms. The value of electronegativity for the xenon atom is 2.6 while that oxygen atom is 3.44 and fluorine has the highest electronegativity value of 3.98. Therefore, as all the bonds in this molecule are polar and also, it has an asymmetric shape due to which the dipole moment does not get canceled and the XeO2F2 molecule is polar.
Conclusion
The Lewis structure of the XeO2F2 molecule is :
The electron geometry of the XeO2F2 molecule is trigonal bipyramidal while its molecular geometry is a see-saw. The hybridization state of the XeO2F2 molecule is dsp3. The XeO2F2 molecule is polar.