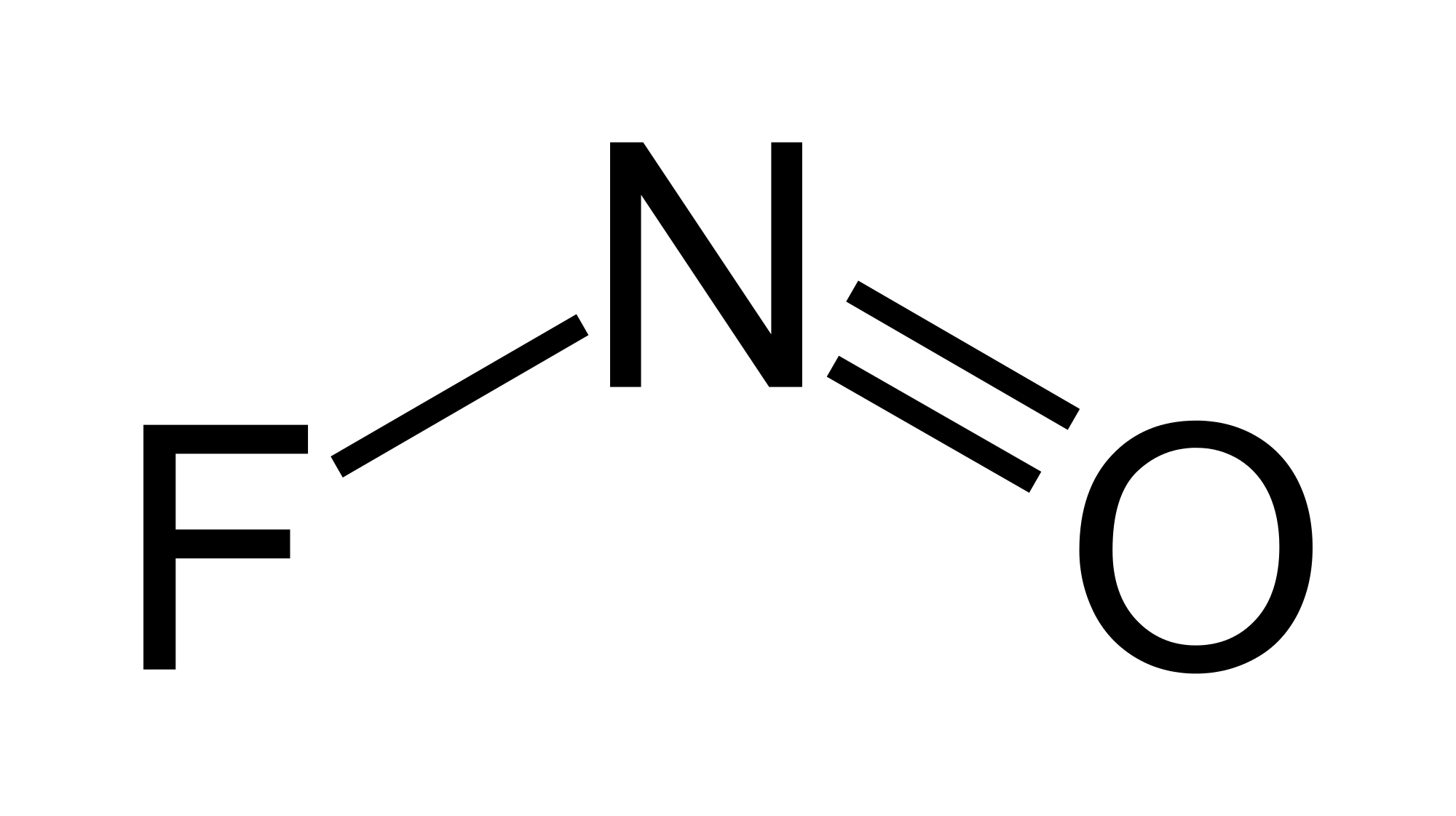
It is used for the conversion of metals to their fluorides and alcohols to nitrites. It is also a proposed oxidizer for rocket propellants. In this article, we will study the lewis structure of NOF, its hybridization, polarity, and geometry. So before directly going over it. Let’s get familiar with the below terminologies.
Valence Electrons and Octet Rule
The electrons present in the outermost shell of an atom are called valence electrons. These are majorly responsible for the characteristics displayed by any atom and play a very important role in chemical bonding. Gilbert Lewis deduced that the atoms are most stable when the number of valence electrons is equal to eight, this is known as the octet rule. It further states that every atom tends to complete its octet by the formation of a bond with other atoms either by sharing or exchange of electrons. However, Helium is an exception to this rule which is satisfied with two valence electrons and also derives the basis of stability of the H2 atom. Few properties of Nitrosyl Fluoride are listed in the table below.
Lewis Structure of NOF
Lewis structure as we already know is the pictorial representation of electrons around the atoms in a molecule. The basic idea is to draw the most stable structure possible for a molecule with the least inter-electronic repulsion. It was introduced in 1916 by Gilbert N. Lewis. The Lewis structure for NOF is:
As discussed earlier atoms are most stable when their octet is complete. Looking at the above structure it is clear that the octet of all the three atoms involved is satisfied and the lone pairs are placed as far apart as possible, indicating this to be the most precise lewis structure for NOF.
Drawing Lewis Structure of NOF
Step 1: To draw the Lewis structure of NOF we first need to choose a central atom. As nitrogen is the least electronegative element amongst all the three atoms involved it is chosen as the central atom. The Oxygen and Fluorine atoms are placed on each side of the Nitrogen atom. Step 2: Now counting the number of valence electrons in the molecule: For Nitrogen (Group 15 element), number of valence electron = 5 For Oxygen (Group 16 element), number of valence electron = 6 For Fluorine (Group 17 element), number of valence electron = 7 Now counting total number of valence electron= 5 + 6 + 7 = 18 valence electrons Step 3: Thereafter, the valence electrons of all the three atoms inside the molecule are placed around them in the form of dots, each dot in the structure represents an electron, and the three atoms are connected with the single bonds. Step 4: Looking at the structure and distribution of electrons in the molecule it can be seen that the octet of both fluorine and oxygen is complete, however, Nitrogen is in short of two electrons. Step 5: As Fluorine is the most electronegative out of all the three atoms involved in the formation of the molecule it does not share its electron with Nitrogen. Thus, Nitrogen forms a double by sharing two electrons with Oxygen after redistribution of electrons.
Step 6: Finally, by completing the octet of all the atoms, the lewis structure appears to be like this:
From the above structure, it is clear that the octet for all the atoms involved in the formation of the molecule is satisfied. Nitrogen, Oxygen, and Fluorine atoms are left with one, two, and three lone pairs of electrons, respectively.
Formal Charge
The formal charge is the theoretical concept devised to establish the efficiency of the derived Lewis structure. The structure for which formal charge is closest to zero is considered correct. This charge is assigned based on the assumption that electrons are distributed equally inside a molecule. It is calculated for individual atoms. The formula for Formal charge is given as:
For calculating the formal charge for NOF, each atom is considered individually. • For N: Total number of the valence electrons in Free State = 5 Total number of non-bonding electron = 2 Total number of bonding electrons = 6 Putting these values in above formula, Formal Charge = 5 – [ 2 – 6/2] = 0 • For O: Total number of valence electron in Free State = 6 Total number of non-bonding electron = 4 Total number of bonding electron = 4 Putting these values in above formula, Formal Charge = 6 – [ 4 – 4/2] = 0 • Similarly for F: Total number of valence electron in Free State = 7 Total number of non-bonding electron = 6 Total number of bonding electron = 2 Putting these values in above formula, Formal Charge = 7 – [ 6 – 2/2] = 0 Therefore, the total formal charge on the molecule also becomes zero indicating that the derived Lewis structure is correct.
Molecular Geometry of NOF
The Valence Shell Electron Pair Repulsion (VSEPR) Theory clearly states that electrons inside a molecule tend to arrange themselves in a manner to avoid inter-electronic repulsion. This repulsion exists between bonding as well as non-bonding electrons (lone pairs). However, it is the maximum between lone pairs of electrons due to the available free space around them. Therefore, the shape of a molecule is determined by the number of lone pairs present as well as the extent of electronic repulsion present in the molecule. Also, the lone pair of electrons present upon the central atom determines the distortion of the bond angle between the central atom and other atoms. So, to comprehend the molecular geometry of NOF, as per the VSEPR theory, we will first have to choose a central atom. Being least electronegative nitrogen occupies this position, which is bonded to Oxygen and Fluorine atoms through the double and triple bonds, respectively. As clear from the Lewis structure of NOF, three regions of electron density are available around nitrogen atom viz. one lone pair, one double bond, and one single bond. Therefore, the electron pair geometry here would be trigonal planar with a bond angle of about 120°.
However, as molecular geometry focuses on the location of atoms in space and also as discussed earlier there is one lone pair of electrons attached with the Nitrogen atom which forces both the groups present on the central atom downwards. This results in distortion of the molecule, resulting in a bent structure with a bond angle of approximately 110°.
H2O molecule also has a bent-shaped structure. Check out the article I wrote on lewis structure, hybridization, geometry of H2O.
Hybridization of NOF
The theory of hybridization was first proposed by Linus Pauling in 1931. As the name indicates hybridization refers to the mixing of two or more orbitals such as s, p, d, f, etc. thereby forming a hybrid orbital. The only condition here is that the orbital should be of similar energy. The hybrid orbital is named after the individual atomic orbital that comes together for its formation. For example, the sp3 orbital indicates that one s and 3 p orbitals were mixed for its formation. Hybridization = Number of sigma (σ) bond on central atom + lone pair on the central atom Determining the hybridization for NOF: Number of sigma bond on central atom = 2 Number of lone pair on central atom = 1 Therefore, Hybridization = 2 + 1 = 3 i.e. sp2 hybridization Further, the electron pair geometry of NOF is trigonal planar indicating sp2 hybridization. Also, the p-orbital is perpendicular to the plane which is a characteristic of sp2 hybridization. Taking a look at the bonding of NOF molecule:
From the above structure, it is clear that Nitrogen is bonded to Oxygen through a double bond i.e. sp2 hybridization. And on the other side Nitrogen is bonded to Fluorine through a single bond i.e. sp3 hybridization but owing to the presence of lone pair, which acts as a pi bond the hybridization here also becomes sp2 hence, confirming that NOF is sp2 hybridized.
Polarity of NOF
Polarity refers to the presence of two opposite charges viz. positive and negative, on different atoms of the same molecule. This usually occurs due to the difference in electronegativity of combining atoms resulting in the formation of polar bonds. The partial charges on dissimilar atoms are the small electric charges, which signify the occurrence of a polar bond. Owing to the charges developed on the molecule due to the electronegativity difference between Oxygen and Fluorine, and also the charge due to the presence of lone pair of the electron the dipole moment of NOF is 1.837 D.
Conclusion
• The formal charge on the NOF molecule is 0. • Electron pair geometry for NOF is trigonal planar with a bond angle of about 120°. • Molecular Geometry for NOF is bent with a bond angle of 110°. • NOF molecule is sp2 hybridized. • The Dipole Moment of the NOF molecule is 1.837 D. I hope you guys found the article informative. If you want me to write on any specific topic, your suggestions are always welcomed. Let me know in case you have any queries. Thanks !!